LYME TRANSMISSION: Rhipicephalus and Dermecentor Ticks
|
Including examination of I. uriae (seabird)
& I. auritulus (bird) ticks |
Including examination of H. longicornis
(scrub/bush) & H. bispinosa ticks |
Examination of Rhipicephalus & Dermecentor Ticks in the Borrelia cycle
The presence of Lyme disease continues to be denied in Australia due to one study conducted in 1994 and the fact that Australia does not have any of the first four ticks that were initially identified as vectors of Lyme disease in the Northern hemisphere. As has been outlined in the Transmission and Maintenance section / Tick Vector Table, continued global research has shown the number of ticks implicated in the Borrelia cycle is much larger.
There are a number of ticks present in Australia that have since been identified as tick vectors (as discussed in Tick Vectors & Reservoir Hosts section) as well as those not listed on the Tick Vector Table that are implicated in maintaining the Borrelia cycle and require much further research. These include ticks from the Rhipicephalus and Dermacentor family.
There are a number of ticks present in Australia that have since been identified as tick vectors (as discussed in Tick Vectors & Reservoir Hosts section) as well as those not listed on the Tick Vector Table that are implicated in maintaining the Borrelia cycle and require much further research. These include ticks from the Rhipicephalus and Dermacentor family.
Rhipicephalus Ticks: R. sanguineus and R. Microplus: These tick species are found in Australia. They have been implicated in the Borrelia cycle, in that they have been found to carry the Borrelia spirochete and are therefore possible vectors of Borrelia.
Dermacentor Ticks: Although this family of ticks is not in Australia, this species is briefly mentioned in order to demonstrate that when looking at the vector competence of a particular species of ticks that findings on competence may be altered when ticks are examined in co-feeding studies (numerous tick species feeding together - which would emulate the natural environment), as opposed to ‘traditional laboratory’ studies where only one tick species is commonly examined.
For example, while ticks of the Dermencentor species may be found to be incompetent vectors when feeding alone, in studies where they are co-fed with other species of ticks, they are found to be competent vectors of Borrelia. Considering that in the natural environment many different species of ticks may be found on the host animal, further co-feeding studies of various tick species are essential.
Dermacentor Ticks: Although this family of ticks is not in Australia, this species is briefly mentioned in order to demonstrate that when looking at the vector competence of a particular species of ticks that findings on competence may be altered when ticks are examined in co-feeding studies (numerous tick species feeding together - which would emulate the natural environment), as opposed to ‘traditional laboratory’ studies where only one tick species is commonly examined.
For example, while ticks of the Dermencentor species may be found to be incompetent vectors when feeding alone, in studies where they are co-fed with other species of ticks, they are found to be competent vectors of Borrelia. Considering that in the natural environment many different species of ticks may be found on the host animal, further co-feeding studies of various tick species are essential.
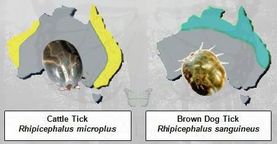
Rhipicephalus Ticks : R. Sanguineus (Brown Dog Tick) and R. Microplus (Cattle Tick)
Brown Dog Tick: Rhipicephalus sanguineus
R. sanguineus, or the brown dog tick, is located worldwide. In Australia, it is verified as present in every state apart from Tasmania (1: CSRIO info, last updated 2004). It is a tick of “great medical and veterinary significance being the vector and reservoir of many human and animal pathogens” (2: pge 349). Human pathogens include Bartonella, several species of Rickettsia, and Coxiella burnetii (Q fever). Animal pathogens include; Ehrlichia canis, several Babesia species such as Canis vogelli and gibsoni and it is a suspected vector of Anaplasma (2-4). It is also involved in the transmission of Theileria (a protozoa that is closely related to Babesia) species such as Theileria parva, otherwise known as East Coast Fever and Theileria ovis (5,6).
Vector competence has not been established with regards to Borrelia, although it has been found to harbour Borrelia in both America (7) and Europe (8). It is also the suspected vector in Mexico, where a 2008 study in Mexicali, Baja California (a Mexico-US Border City) reported “the existence of B.burgdorferi past/present infection in dogs in an area where the only identified tick is R. sanguineus” (9). This species should be examined both for the Borrelia species they may carry and their vector capabilities.
Vector competence has not been established with regards to Borrelia, although it has been found to harbour Borrelia in both America (7) and Europe (8). It is also the suspected vector in Mexico, where a 2008 study in Mexicali, Baja California (a Mexico-US Border City) reported “the existence of B.burgdorferi past/present infection in dogs in an area where the only identified tick is R. sanguineus” (9). This species should be examined both for the Borrelia species they may carry and their vector capabilities.
Cattle Tick: Rhipicephalus microplus
R. microplus (previously known as Boophilus microplus), otherwise known as the cattle tick, is considered the most important parasite of livestock in the world (10). It was first introduced into Australia (Darwin) in 1872 on cattle from Indonesia. By 1895 it had spread to Western Australia, reaching Queensland in 1891 and New South Wales in 1906 (11). This tick differs from all other ticks mentioned in the Borrelia cycle, in that it is a one host (rather than three host) tick, meaning that it spends its entire life (much shorter cycle than other ticks also) on the one host. As the name suggests, the primary hosts of this tick are cattle, though it may also be found on horses, sheep, goats, camels, alpacas, llamas, deer and dogs (10, 12,13). Although not a common occurrence, these ticks may also attach to humans who come into contact with them (10, 12, 14). While it may not come into contact with humans on a regular basis, this tick may serve to keep the Borrelia cycle active within the environment.
Borrelia burgdorferi has been isolated from R. microplus (14-16), though it ability as a vector of this species of Borrelia is unclear. It is however a known vector of Borrelia Theileri, the species responsible for bovine borreliosis. “To date, only B. burgdorferi ss and B. garinii have been described in bovine Lyme disease. However, two other spirochetes, B. theileri and B. coriaceae have been described in cattle and considered as the agent of bovine borreliosis and as the putative agent of epizootic bovine abortion, respectively” (17:pg 2). B. theileri has been noted in Australian cattle for over 50 years (18).
DNA sequencing reveals that B. theileri is in the same clade as B. lonestari and B. miyamotoi, the species of Borrelia that are responsible for relapsing fever/ lyme-like disease in humans (19, 20). Indeed, they are that similar it has been postulated that due to the eradication of the R. microplus ticks from America, the lonestari Borrelia species that is found in the A. americanum tick may have originally been due to the Borrelia theileri bacteria relocating from the R. microplus tick to the A. americanum (14).
With the presence of B. theileri in Australia, combined with the possibility of host shifting and adaptation of various Borrelia species, along with the importations of cattle from countries where Lyme is endemic, further investigations of R. Microplus ticks to ascertain what pathogens they carry and whether they are infectious to humans is certainly warranted.
Borrelia burgdorferi has been isolated from R. microplus (14-16), though it ability as a vector of this species of Borrelia is unclear. It is however a known vector of Borrelia Theileri, the species responsible for bovine borreliosis. “To date, only B. burgdorferi ss and B. garinii have been described in bovine Lyme disease. However, two other spirochetes, B. theileri and B. coriaceae have been described in cattle and considered as the agent of bovine borreliosis and as the putative agent of epizootic bovine abortion, respectively” (17:pg 2). B. theileri has been noted in Australian cattle for over 50 years (18).
DNA sequencing reveals that B. theileri is in the same clade as B. lonestari and B. miyamotoi, the species of Borrelia that are responsible for relapsing fever/ lyme-like disease in humans (19, 20). Indeed, they are that similar it has been postulated that due to the eradication of the R. microplus ticks from America, the lonestari Borrelia species that is found in the A. americanum tick may have originally been due to the Borrelia theileri bacteria relocating from the R. microplus tick to the A. americanum (14).
With the presence of B. theileri in Australia, combined with the possibility of host shifting and adaptation of various Borrelia species, along with the importations of cattle from countries where Lyme is endemic, further investigations of R. Microplus ticks to ascertain what pathogens they carry and whether they are infectious to humans is certainly warranted.
Various pathogens carried by R. Microplus
Along with its role in various Borrelia species (ie: found to harbour B. burgdorferi and is a competent vector of Bovine Borrelia) R. microplus is the vector for many zoonotic pathogens; including those responsible for “Tick Fever”; Babesia bovis, B. bigemina and Anaplasma marginale, which may result in sickness and death in cattle (30-33) as well as humans, particularly those that are immune-compromised (30, 34). It is also suspected as a vector of Theileria equi (30), previously known as Babesia equi, and has been found to carry Ehrlichia, Wolbachia, and Coxiella burnetti (33).
It is long overdue that the health departments in Australia communicate information acknowledged in the rest of the world by updating the information such as that found on the Queensland Government: Agriculture, Fisheries and Forestry website: “People can find cattle tick on themselves after working with cattle or other animals. The ticks are easily removed and cause no lasting affect apart from the site itching for a few days” (31).
It urgently needs to be acknowledged that the Babesia parasites these ticks can carry can be passed on to humans and result in clinical illness. Babesia bovis and bigemina may have only been implicated in a small number of cases of human Babesiosis, but that possibility is there, as is the potential to transmit any other species / pathogens that the ticks may carry such as Coxiella burnetti, the pathogen underlying Q fever.
The Haemaphysalis longicornis (Bush & Cattle Tick) is also discussed with regards to the various pathogens it carries, including Borrelia and Babesia:
Click Here (and scroll through to bottom of the page)
For more on Babesia in Australia and for Clinical Symptoms Click Here
Along with its role in various Borrelia species (ie: found to harbour B. burgdorferi and is a competent vector of Bovine Borrelia) R. microplus is the vector for many zoonotic pathogens; including those responsible for “Tick Fever”; Babesia bovis, B. bigemina and Anaplasma marginale, which may result in sickness and death in cattle (30-33) as well as humans, particularly those that are immune-compromised (30, 34). It is also suspected as a vector of Theileria equi (30), previously known as Babesia equi, and has been found to carry Ehrlichia, Wolbachia, and Coxiella burnetti (33).
It is long overdue that the health departments in Australia communicate information acknowledged in the rest of the world by updating the information such as that found on the Queensland Government: Agriculture, Fisheries and Forestry website: “People can find cattle tick on themselves after working with cattle or other animals. The ticks are easily removed and cause no lasting affect apart from the site itching for a few days” (31).
It urgently needs to be acknowledged that the Babesia parasites these ticks can carry can be passed on to humans and result in clinical illness. Babesia bovis and bigemina may have only been implicated in a small number of cases of human Babesiosis, but that possibility is there, as is the potential to transmit any other species / pathogens that the ticks may carry such as Coxiella burnetti, the pathogen underlying Q fever.
The Haemaphysalis longicornis (Bush & Cattle Tick) is also discussed with regards to the various pathogens it carries, including Borrelia and Babesia:
Click Here (and scroll through to bottom of the page)
For more on Babesia in Australia and for Clinical Symptoms Click Here
Dermacentor Species
Borrelia has been found in ticks of the Dermacentor genera, though similar to the Rhipicephalus genera, their competence as vectors is controversial and requires further investigation. The controversy with regards to the Dermacentor species lays in the fact that while ticks of this species may be found to be incompetent vectors when feeding alone, in studies where they are co-fed with other species of ticks, they are found to be competent vectors. Although there are no ticks of the Dermacentor genera in Australia, this family of ticks is examined briefly below due to the significance of these findings.
Species from the Dermecentor genera include those found in
America: D. variabilis (American Dog Tick) and D. andersoni (Rocky Mountain Wood Tick), and
Europe/Asia: D. reticulates (Marsh tick or Ornate cow tick) and D. marginatus (Ornate sheep tick).
America: Borrelia has been found in both D. andersoni (Rocky Mountain Wood Tick) (1) and D. variabilis (American Dog Tick) (1- 5). Whilst this indicates their ability to acquire infection from a host animal, whether they maintain that infection through their next molt / life cycle, or are able to pass it on to another host is unknown. Studies on Dermacentor ticks are mixed: When the tick is examined in isolation, it is not considered/found to be a competent vector, however, when “they feed in conjunction with Ixodes scapularis ticks, the Dermacentor ticks can acquire and transmit Borrelia burgdorferi sensu stricto” (6). The combination of different salivary factors of the ticks feeding in close proximity is believed to be the underlying factor in this finding.
Europe / Asia: Two Dermacentor species found in Europe / Asia are the D. reticulates (Marsh or Ornate cow tick) and the D. marginatus (Ornate sheep tick). Both species may feed on humans, particularly the scalp (7), and both have been found to harbour Borrelia (8-10). D. reticulates has been suggested to be involved in the transmission cycle of Borrelia in Europe (11) and a case of human Lyme disease after the bite of a D. marginatus in Bulgaria has been reported (12).
Considering that in the natural environment many different species of ticks may be found on the host animal, further co-feeding studies of various tick species are warranted and urgently required to further understand the co-feeding phenomenon revealed through examination of the Dermecentor genera.
Species from the Dermecentor genera include those found in
America: D. variabilis (American Dog Tick) and D. andersoni (Rocky Mountain Wood Tick), and
Europe/Asia: D. reticulates (Marsh tick or Ornate cow tick) and D. marginatus (Ornate sheep tick).
America: Borrelia has been found in both D. andersoni (Rocky Mountain Wood Tick) (1) and D. variabilis (American Dog Tick) (1- 5). Whilst this indicates their ability to acquire infection from a host animal, whether they maintain that infection through their next molt / life cycle, or are able to pass it on to another host is unknown. Studies on Dermacentor ticks are mixed: When the tick is examined in isolation, it is not considered/found to be a competent vector, however, when “they feed in conjunction with Ixodes scapularis ticks, the Dermacentor ticks can acquire and transmit Borrelia burgdorferi sensu stricto” (6). The combination of different salivary factors of the ticks feeding in close proximity is believed to be the underlying factor in this finding.
Europe / Asia: Two Dermacentor species found in Europe / Asia are the D. reticulates (Marsh or Ornate cow tick) and the D. marginatus (Ornate sheep tick). Both species may feed on humans, particularly the scalp (7), and both have been found to harbour Borrelia (8-10). D. reticulates has been suggested to be involved in the transmission cycle of Borrelia in Europe (11) and a case of human Lyme disease after the bite of a D. marginatus in Bulgaria has been reported (12).
Considering that in the natural environment many different species of ticks may be found on the host animal, further co-feeding studies of various tick species are warranted and urgently required to further understand the co-feeding phenomenon revealed through examination of the Dermecentor genera.
References: LYME TRANSMISSION: Rhipicephalus and Dermecentor Ticks
Please note: Any information with regards to Lyme disease that is freely available at numerous locations on the internet has not been referenced. For specific facts/arguments, see the reference list.
NB: Reference section is separated into segments for ease of updating information
NB: Reference section is separated into segments for ease of updating information
Rhipicephalus Ticks
Brown Dog Tick: Rhipicephalus sanguineus
(1) CSRIO, Rhipicephalus sanguineus: http://www.ces.csiro.au/aicn/system/c_129.htm
(2) Igor Uspensky (2008) Ticks (Acari: Ixodoidea) as Urban Pests and Vectors with Special Emphasis on Ticks Outside their Geographical Range. Proceedings of the Sixth International Conference on Urban Pests
William H Robinson and Dániel Bajomi (editors). Printed by OOK-Press Kft., H-8200 Veszprém, Pápai út 37/a, Hungary http://www.icup.org.uk/reports%5CICUP893.pdf
(3) Table 1 : Major canine vector-borne diseases: Day Parasites & Vectors 2011 4:48 doi:10.1186/1756-3305-4-48 http://www.parasitesandvectors.com/content/4/1/48/table/T1
(4) Boozer L and Macintire D (2005) Babesia gibsoni: An Emerging Pathogen in Dogs. Compendium
http://cp.vetlearn.com/Media/PublicationsArticle/PV_27_01_33.pdf
(5) Infection With Various Protozoa: Babesia. Distance Learning Lecture Notes.
http://www.itg.be/itg/distancelearning/lecturenotesvandenendene/08_Various_protozoap13.htm
(6) Zakkyeh T, Mohammad Ali O, Nasibeh HV, Mohammad Reza YE, Farhang B, and Fatemeh M (2012). First molecular detection of Theileria ovis in Rhipicephalus sanguineus tick in Iran. Asian Pac J Trop Med: 5(1):29-32. http://www.ncbi.nlm.nih.gov/pubmed/22182639
(7) Canadian Lyme Disease Foundation; Tick Vectors: http://www.canlyme.com/ticks.html
(8) Hubbard MJ, Baker AS and Cann KJ (1998) Distribution of Borrelia burgdorferi s.l. spirochaete DNA in British ticks (Argasidae and Ixodidae) since the 19th Century, assessed by PCR. Medical and Veterinary Entomology, 12: 89–97. http://www.ncbi.nlm.nih.gov/pubmed/9513944
(9) Tinoco-Gracia L, Quiroz-Romero H, Quintero-Martinez MT, Renteria-Evangelista TB, Barreras-Serrano A, Hori-Oshima S, Medina-Basulto G, Vinasco J and Moro MH (2008) Prevalence and Risk Factors for Borrelia burgdorferi Infection in Mexicali, Baja California, a Mexico-US Border City. Intern J Appl Res Vet Med. 6(3) 161-165. http://www.jarvm.com/articles/Vol6Iss3/Tinoco_GraciaVol6Iss3161-165.pdf
Cattle Tick: Rhipicephalus microplus
(10) Rhipicephalus (Boophilus) microplus. The Centre for Food Security and Public Health. Iowa State University. College of Veterinary Medicine: http://www.cfsph.iastate.edu/Factsheets/pdfs/boophilus_microplus.pdf
(11) CSRIO, Rhipicephalus Microplus: http://www.ces.csiro.au/aicn/system/c_112.htm
(12) Queensland Government: Agriculture, Fisheries and Forestry: http://www.daff.qld.gov.au/4790_12815.htm Accessed 28th July 2012
Updated: 13th November 2012 - https://www.daf.qld.gov.au/animal-industries/animal-health-and-diseases/animal-disease-control/cattle-tick/overview
(13) Pfizer Animal Health, Cattle Tick: https://www.pfizeranimalhealth.com.au/diseases/417/cattle-tick.aspx
Accessed 28th July 2012
(14) Andreotti R, Perez de Leon AA, Dowd SE, Guerrero FD, Bendele KG and Scoles GA (2011). Assessment of bacterial diversity in the cattletick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol;11(1):6. http://www.ncbi.nlm.nih.gov/pubmed/21211038 : http://www.biomedcentral.com/1471-2180/11/6
(15) Development of Three Detection Techniques for Borrelia Burgdorferi Sensu Lato Agricultural Science Research. March 2012 : http://www.agrpaper.com/development-of-three-detection-techniques-for-borrelia-burgdorferi-sensu-lato.htm
(16) Chu CY, Jiang BG, Liu W, Zhao QM, Wu XM, Zhang PH, Zhan H and Cao WC (2008). Presence of pathogenic Borrelia burgdorferi sensu lato in ticks and rodents in Zhejiang, south-east China. J Med Microbiol;57( 8):980-5 http://www.ncbi.nlm.nih.gov/pubmed/18628499
(17) Boulouis H-J, Maillard R and Haddad R. Lyme Borreliosis in Cattle. World Buiatrics Congress 2006. Nice, France. http://www.ivis.org/proceedings/wbc/wbc2006/boulouis.pdf
(18) Callow LL (1967) Observations on tick-transmitted spirochaetes of cattle in Australia and South Africa. Br Vet J 1967 Nov;123(11):492-7. http://www.ncbi.nlm.nih.gov/pubmed/6070621
(19) Rich SM, Armstrong PM, Smith RD and Telford SR 3rd (2001) Lone star tick-infecting borreliae are most closely related to the agent of bovine borreliosis. J Clin Microbiol: 39(2):494-7. http://www.ncbi.nlm.nih.gov/pubmed/11158095
(20) Yparraguirre LA, Machado-Ferreira E, Ullmann AJ, Piesman J, Zeidner NS and Soares CA (2007) A hard tick relapsing fever group spirochete in a Brazilian Rhipicephalus (Boophilus) microplus. Vector Borne Zooonotic Dis; 7(4):717-21. http://www.ncbi.nlm.nih.gov/pubmed/17979536
Various pathogens carried by R. Microplus
(30) Rhipicephalus (Boophilus) microplus. The Centre for Food Security and Public Health. Iowa State University. College of Veterinary Medicine: http://www.cfsph.iastate.edu/Factsheets/pdfs/boophilus_microplus.pdf
(31) Queensland Government: Agriculture, Fisheries and Forestry: http://www.daff.qld.gov.au/4790_12815.htm Accessed 28th July 2012
Updated: 13th November 2012 - https://www.daf.qld.gov.au/animal-industries/animal-health-and-diseases/animal-disease-control/cattle-tick/overview
(32) Pfizer Animal Health, Cattle Tick: https://www.pfizeranimalhealth.com.au/diseases/417/cattle-tick.aspx
Accessed 28th July 2012
(33) Andreotti R, Perez de Leon AA, Dowd SE, Guerrero FD, Bendele KG and Scoles GA (2011). Assessment of bacterial diversity in the cattletick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol;11(1):6. http://www.ncbi.nlm.nih.gov/pubmed/21211038 : http://www.biomedcentral.com/1471-2180/11/6
(34) Mylonakis E (2001) When to Suspect and How to Monitor Babesiosis. Am Fam Physician; 15;63(10):1969-1975. http://www.aafp.org/afp/2001/0515/p1969.html
Dermecentor Ticks
(1) Canadian Lyme Disease Foundation; Tick Vectors: http://www.canlyme.com/ticks.html
(2) Ouellette J, Apperson CS, Howard P, Evans TL and Levine JF( 1997) Tick-raccoon associations and the potential for Lyme disease spirochete transmission in the coastal plain of North Carolina. J Wildl Dis; 33(1):28-39. http://www.ncbi.nlm.nih.gov/pubmed/9027688 http://www.jwildlifedis.org/content/33/1/28.abstract
(3) Williamson PC, Billingsley PM, Teltow GJ, Seals JP, Turnbough MA and Atkinson SF (2010). Borrelia, Ehrlichia, and Rickettsia spp. in ticks removed from persons, Texas, USA. Emerg Infect Dis; 16 (3) 441-446. http://wwwnc.cdc.gov/eid/article/16/3/09-1333_article.htm
(4) Lin T, Gao L, Seyfang A and Oliver JH Jr (2005) 'Candidatus Borrelia texasensis', from the American dog tick Dermacentor variabilis. Int J Syst Evol Microbiol; ;55(Pt 2):685-93. http://www.ncbi.nlm.nih.gov/pubmed/15774644 http://ijs.sgmjournals.org/content/55/2/685.full
(5) Magnarelli LA and Anderson JF (1988) Ticks and Biting Insects Infected with the Etiologic Agent of Lyme Disease, Borrelia burgdorferi. J Clin Microbiol: 26 (8): 1482-6. http://www.ncbi.nlm.nih.gov/pubmed/3170711
(6) Masters EJ, Grigery CN and Masters RW (2008) STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect Dis Clin North Am; 22(2):361-76. http://www.ncbi.nlm.nih.gov/pubmed/18452807
(7) Parola P and Raoult D (2001) Ticks and Tickborne Bacterial Diseases in Humans: An Emerging Infectious Threat. Clin Infect Dis; 32(6): 897-928. http://cid.oxfordjournals.org/content/32/6/897.full
(8) Kahl O, Janetzki C, Gray JS, Stein J and Bauch RJ (1992) Tick infection rates with Borrelia: Ixodes ricinus versus Haemaphysalis concinna and Dermacentor reticulatus in two locations in eastern Germany. Med Vet Entomol;6(4):363-6. http://www.ncbi.nlm.nih.gov/pubmed/1463902
(9) Angelov L, Dimova P and Berbencova W (1996) Clinical and laboratory evidence of the importance of the tick D. marginatus as a vector of B. burgdorferi in some areas of sporadic Lyme disease in Bulgaria. Eur J Epidemiol; 12(5):499-502. http://www.ncbi.nlm.nih.gov/pubmed/8905312 http://www.jstor.org/pss/3581664
(10) Hubbard MJ, Baker AS and Cann KJ (1998) Distribution of Borrelia burgdorferi s.l. spirochaete DNA in British ticks (Argasidae and Ixodidae) since the 19th Century, assessed by PCR. Medical and Veterinary Entomology, 12: 89–97. http://www.ncbi.nlm.nih.gov/pubmed/9513944
(11) Biadun W, Rzymowska J, Stephien-Rukasz H, Niemczyk M and Chybowski J (2007) Occurrence of Borrelia Burgdoreri Sensu Lato in Ixodes Ricinus and Dermacentor Reticulatus Ticks Collected From Roe Deer and Deer Shot in the South-East of Poland. Bull Vet Inst Pulawy; 51, 213-217. http://lymepoland.com/pliki/05_biadun.pdf
(12) Angelov L, Dimova P and Berbencova W (1996) Clinical and laboratory evidence of the importance of the tick D. marginatus as a vector of B. burgdorferi in some areas of sporadic Lyme disease in Bulgaria. Eur J Epidemiol; 12(5):499-502 http://www.ncbi.nlm.nih.gov/pubmed/8905312
Brown Dog Tick: Rhipicephalus sanguineus
(1) CSRIO, Rhipicephalus sanguineus: http://www.ces.csiro.au/aicn/system/c_129.htm
(2) Igor Uspensky (2008) Ticks (Acari: Ixodoidea) as Urban Pests and Vectors with Special Emphasis on Ticks Outside their Geographical Range. Proceedings of the Sixth International Conference on Urban Pests
William H Robinson and Dániel Bajomi (editors). Printed by OOK-Press Kft., H-8200 Veszprém, Pápai út 37/a, Hungary http://www.icup.org.uk/reports%5CICUP893.pdf
(3) Table 1 : Major canine vector-borne diseases: Day Parasites & Vectors 2011 4:48 doi:10.1186/1756-3305-4-48 http://www.parasitesandvectors.com/content/4/1/48/table/T1
(4) Boozer L and Macintire D (2005) Babesia gibsoni: An Emerging Pathogen in Dogs. Compendium
http://cp.vetlearn.com/Media/PublicationsArticle/PV_27_01_33.pdf
(5) Infection With Various Protozoa: Babesia. Distance Learning Lecture Notes.
http://www.itg.be/itg/distancelearning/lecturenotesvandenendene/08_Various_protozoap13.htm
(6) Zakkyeh T, Mohammad Ali O, Nasibeh HV, Mohammad Reza YE, Farhang B, and Fatemeh M (2012). First molecular detection of Theileria ovis in Rhipicephalus sanguineus tick in Iran. Asian Pac J Trop Med: 5(1):29-32. http://www.ncbi.nlm.nih.gov/pubmed/22182639
(7) Canadian Lyme Disease Foundation; Tick Vectors: http://www.canlyme.com/ticks.html
(8) Hubbard MJ, Baker AS and Cann KJ (1998) Distribution of Borrelia burgdorferi s.l. spirochaete DNA in British ticks (Argasidae and Ixodidae) since the 19th Century, assessed by PCR. Medical and Veterinary Entomology, 12: 89–97. http://www.ncbi.nlm.nih.gov/pubmed/9513944
(9) Tinoco-Gracia L, Quiroz-Romero H, Quintero-Martinez MT, Renteria-Evangelista TB, Barreras-Serrano A, Hori-Oshima S, Medina-Basulto G, Vinasco J and Moro MH (2008) Prevalence and Risk Factors for Borrelia burgdorferi Infection in Mexicali, Baja California, a Mexico-US Border City. Intern J Appl Res Vet Med. 6(3) 161-165. http://www.jarvm.com/articles/Vol6Iss3/Tinoco_GraciaVol6Iss3161-165.pdf
Cattle Tick: Rhipicephalus microplus
(10) Rhipicephalus (Boophilus) microplus. The Centre for Food Security and Public Health. Iowa State University. College of Veterinary Medicine: http://www.cfsph.iastate.edu/Factsheets/pdfs/boophilus_microplus.pdf
(11) CSRIO, Rhipicephalus Microplus: http://www.ces.csiro.au/aicn/system/c_112.htm
(12) Queensland Government: Agriculture, Fisheries and Forestry: http://www.daff.qld.gov.au/4790_12815.htm Accessed 28th July 2012
Updated: 13th November 2012 - https://www.daf.qld.gov.au/animal-industries/animal-health-and-diseases/animal-disease-control/cattle-tick/overview
(13) Pfizer Animal Health, Cattle Tick: https://www.pfizeranimalhealth.com.au/diseases/417/cattle-tick.aspx
Accessed 28th July 2012
(14) Andreotti R, Perez de Leon AA, Dowd SE, Guerrero FD, Bendele KG and Scoles GA (2011). Assessment of bacterial diversity in the cattletick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol;11(1):6. http://www.ncbi.nlm.nih.gov/pubmed/21211038 : http://www.biomedcentral.com/1471-2180/11/6
(15) Development of Three Detection Techniques for Borrelia Burgdorferi Sensu Lato Agricultural Science Research. March 2012 : http://www.agrpaper.com/development-of-three-detection-techniques-for-borrelia-burgdorferi-sensu-lato.htm
(16) Chu CY, Jiang BG, Liu W, Zhao QM, Wu XM, Zhang PH, Zhan H and Cao WC (2008). Presence of pathogenic Borrelia burgdorferi sensu lato in ticks and rodents in Zhejiang, south-east China. J Med Microbiol;57( 8):980-5 http://www.ncbi.nlm.nih.gov/pubmed/18628499
(17) Boulouis H-J, Maillard R and Haddad R. Lyme Borreliosis in Cattle. World Buiatrics Congress 2006. Nice, France. http://www.ivis.org/proceedings/wbc/wbc2006/boulouis.pdf
(18) Callow LL (1967) Observations on tick-transmitted spirochaetes of cattle in Australia and South Africa. Br Vet J 1967 Nov;123(11):492-7. http://www.ncbi.nlm.nih.gov/pubmed/6070621
(19) Rich SM, Armstrong PM, Smith RD and Telford SR 3rd (2001) Lone star tick-infecting borreliae are most closely related to the agent of bovine borreliosis. J Clin Microbiol: 39(2):494-7. http://www.ncbi.nlm.nih.gov/pubmed/11158095
(20) Yparraguirre LA, Machado-Ferreira E, Ullmann AJ, Piesman J, Zeidner NS and Soares CA (2007) A hard tick relapsing fever group spirochete in a Brazilian Rhipicephalus (Boophilus) microplus. Vector Borne Zooonotic Dis; 7(4):717-21. http://www.ncbi.nlm.nih.gov/pubmed/17979536
Various pathogens carried by R. Microplus
(30) Rhipicephalus (Boophilus) microplus. The Centre for Food Security and Public Health. Iowa State University. College of Veterinary Medicine: http://www.cfsph.iastate.edu/Factsheets/pdfs/boophilus_microplus.pdf
(31) Queensland Government: Agriculture, Fisheries and Forestry: http://www.daff.qld.gov.au/4790_12815.htm Accessed 28th July 2012
Updated: 13th November 2012 - https://www.daf.qld.gov.au/animal-industries/animal-health-and-diseases/animal-disease-control/cattle-tick/overview
(32) Pfizer Animal Health, Cattle Tick: https://www.pfizeranimalhealth.com.au/diseases/417/cattle-tick.aspx
Accessed 28th July 2012
(33) Andreotti R, Perez de Leon AA, Dowd SE, Guerrero FD, Bendele KG and Scoles GA (2011). Assessment of bacterial diversity in the cattletick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol;11(1):6. http://www.ncbi.nlm.nih.gov/pubmed/21211038 : http://www.biomedcentral.com/1471-2180/11/6
(34) Mylonakis E (2001) When to Suspect and How to Monitor Babesiosis. Am Fam Physician; 15;63(10):1969-1975. http://www.aafp.org/afp/2001/0515/p1969.html
Dermecentor Ticks
(1) Canadian Lyme Disease Foundation; Tick Vectors: http://www.canlyme.com/ticks.html
(2) Ouellette J, Apperson CS, Howard P, Evans TL and Levine JF( 1997) Tick-raccoon associations and the potential for Lyme disease spirochete transmission in the coastal plain of North Carolina. J Wildl Dis; 33(1):28-39. http://www.ncbi.nlm.nih.gov/pubmed/9027688 http://www.jwildlifedis.org/content/33/1/28.abstract
(3) Williamson PC, Billingsley PM, Teltow GJ, Seals JP, Turnbough MA and Atkinson SF (2010). Borrelia, Ehrlichia, and Rickettsia spp. in ticks removed from persons, Texas, USA. Emerg Infect Dis; 16 (3) 441-446. http://wwwnc.cdc.gov/eid/article/16/3/09-1333_article.htm
(4) Lin T, Gao L, Seyfang A and Oliver JH Jr (2005) 'Candidatus Borrelia texasensis', from the American dog tick Dermacentor variabilis. Int J Syst Evol Microbiol; ;55(Pt 2):685-93. http://www.ncbi.nlm.nih.gov/pubmed/15774644 http://ijs.sgmjournals.org/content/55/2/685.full
(5) Magnarelli LA and Anderson JF (1988) Ticks and Biting Insects Infected with the Etiologic Agent of Lyme Disease, Borrelia burgdorferi. J Clin Microbiol: 26 (8): 1482-6. http://www.ncbi.nlm.nih.gov/pubmed/3170711
(6) Masters EJ, Grigery CN and Masters RW (2008) STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect Dis Clin North Am; 22(2):361-76. http://www.ncbi.nlm.nih.gov/pubmed/18452807
(7) Parola P and Raoult D (2001) Ticks and Tickborne Bacterial Diseases in Humans: An Emerging Infectious Threat. Clin Infect Dis; 32(6): 897-928. http://cid.oxfordjournals.org/content/32/6/897.full
(8) Kahl O, Janetzki C, Gray JS, Stein J and Bauch RJ (1992) Tick infection rates with Borrelia: Ixodes ricinus versus Haemaphysalis concinna and Dermacentor reticulatus in two locations in eastern Germany. Med Vet Entomol;6(4):363-6. http://www.ncbi.nlm.nih.gov/pubmed/1463902
(9) Angelov L, Dimova P and Berbencova W (1996) Clinical and laboratory evidence of the importance of the tick D. marginatus as a vector of B. burgdorferi in some areas of sporadic Lyme disease in Bulgaria. Eur J Epidemiol; 12(5):499-502. http://www.ncbi.nlm.nih.gov/pubmed/8905312 http://www.jstor.org/pss/3581664
(10) Hubbard MJ, Baker AS and Cann KJ (1998) Distribution of Borrelia burgdorferi s.l. spirochaete DNA in British ticks (Argasidae and Ixodidae) since the 19th Century, assessed by PCR. Medical and Veterinary Entomology, 12: 89–97. http://www.ncbi.nlm.nih.gov/pubmed/9513944
(11) Biadun W, Rzymowska J, Stephien-Rukasz H, Niemczyk M and Chybowski J (2007) Occurrence of Borrelia Burgdoreri Sensu Lato in Ixodes Ricinus and Dermacentor Reticulatus Ticks Collected From Roe Deer and Deer Shot in the South-East of Poland. Bull Vet Inst Pulawy; 51, 213-217. http://lymepoland.com/pliki/05_biadun.pdf
(12) Angelov L, Dimova P and Berbencova W (1996) Clinical and laboratory evidence of the importance of the tick D. marginatus as a vector of B. burgdorferi in some areas of sporadic Lyme disease in Bulgaria. Eur J Epidemiol; 12(5):499-502 http://www.ncbi.nlm.nih.gov/pubmed/8905312